The WHO Global Patient Safety Action Plan 2021-2030 is a key document that outlines the changes and aspirations that the WHO has for the next decade around protection and wellbeing of patients. The plan, produced on 3 August 2021, aims to educate various stakeholders (NHS, HCPs, pharma and patient groups) about current issues surrounding patient safety and outline their proposed solutions1. The main goal over the next 10 years is a ‘zero harm philosophy’1. This means that although it’s impossible to totally wipe out errors in patient safety, the goal is to work towards being as close to zero harm as possible. But why are we telling you about it now? Because it’s World Patient Safety Day!
“I often say that if health care is not safe, it’s not care.”
– Dr Tedros Adhanom Ghebreyesus, WHO Director-General
Why is patient safety important?
Patient safety affects us all every single day. We will all be ill at some point in our lives and require treatment2, but there are unfortunately occasions where harm can come from this and that is what the WHO are addressing. Patient safety is a new healthcare discipline that emphasizes the reporting, analysis, and prevention of medical errors that often lead to adverse healthcare events3. The resulting knowledge can then be used to educate providers and consumers as well as enhance our current systems to prevent recurrence3.
If patient safety practices are poor, the financial picture is too, with medication errors alone costing 42 billion USD per annum2 – and that’s only one area of accidental patient harm. The impact is even more severe in low-income countries, where patients experience twice as many disability-adjusted life years (DALY) from accidental harm than those in higher income countries (one DALY represents the loss of the equivalent of one year of full health4)2.
“An estimated 2.6 million people die every year in low- and middle- income countries alone due to unsafe care in hospitals […] The vast majority of these deaths – and the costs – are avoidable.”
– Dr Tedros Adhanom Ghebreyesus, WHO Director-General
For our clients, good patient safety is vital for the success of their companies and products. Improving patient safety will help boost repeat custom for products, increase the efficacy and efficiency of drug trials, and make the drugs safer for patients – so everyone benefits! Something we love in patient engagement.
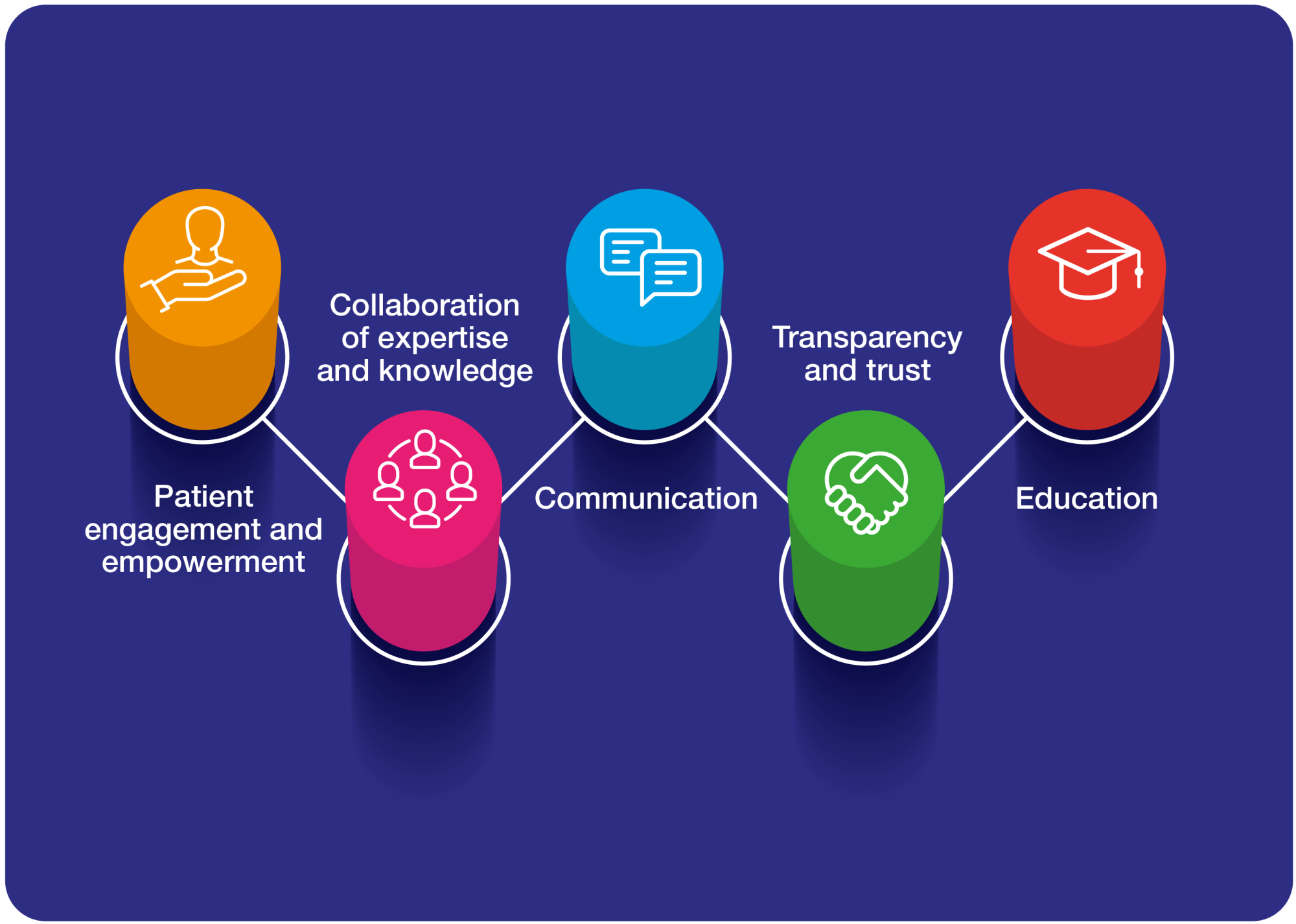
So how does this all relate to patient engagement? From our extensive experience in the field, we know there are 5 key areas where patient safety relates to patient engagement:
- Patient engagement and empowerment
- Collaboration of expertise and knowledge
- Communication
- Transparency and trust
- Education
In each key area, we are exchanging information with patients, collecting data from patient experiences, seeking feedback about recent innovations, and educating each other about how to progress can be made. The success of all 5 relies on trusting relationships with patients that have been built and cultivated in a truly collaborative manner – tokenistic, one-off engagements are not enough to make a sufficient impact.
- Patient engagement and empowerment
 By teaching patients about how their care should be and how to take their medications, they will help us in turn by pointing out errors and shortcomings in care delivery1. One way this will reduce avoidable patient harm is by getting patients to be the eyes and ears of the medical experience to provide insightful feedback. The WHO has created the “Integrated People-Centered Health Services” framework to instigate this and to get patient advocates involved in policy creation, drug development and health schemes1.
By teaching patients about how their care should be and how to take their medications, they will help us in turn by pointing out errors and shortcomings in care delivery1. One way this will reduce avoidable patient harm is by getting patients to be the eyes and ears of the medical experience to provide insightful feedback. The WHO has created the “Integrated People-Centered Health Services” framework to instigate this and to get patient advocates involved in policy creation, drug development and health schemes1.
An empowered patient is a helpful patient. Pharma can help by publicising the need for improvements in patient safety and not only educating patients, but actively providing opportunities (such as advisory boards) for patients to share their insights and by running patient-centric trials5. This will benefit them hugely by directing resources and using their time/money most efficiently. The WHO Global Patient Safety Action Plan 2021-2030 highlights this several times as the key to building a strong foundation for future success in patient safety. This will help to establish a culture of dedication to protecting patient safety, built on a learning loop of listening to patients’ comments, acting on them, and receiving feedback on the changes1. An essential way to make real progress.
The COVID-19 pandemic has engaged the population in healthcare more than ever before, and this newfound enthusiasm should be positively harnessed to improve engagement and understanding in more therapeutic areas1. Over time, this will help to reduce healthcare inequalities, as patients will not only be empowered to understand the care they should be getting, but also to ensure they receive it.
- Collaboration of expertise and knowledge
 Between HCPs, pharma, patient advocates and governing bodies, it’s imperative that knowledge is shared so patient safety practices can grow to their full potential. Furthermore, by ensuring transparency of data around recent incidents/policy changes, we can facilitate faster learning and improvement to achieve a triple win for patients, pharma, and society. Research has shown that most patient safety errors occur due to flawed procedures, processes, and systems – all of which stand a greater chance of being resolved if we share data around what works and what doesn’t2. Once these data are shared, pharma can support the implementation of changes by informing HCPs and patients on lessons learned and steps taken. Pharma companies can promote this unified working by creating networks between themselves, HCPs and patients to help develop a common narrative and raise the profile of patient safety programmes. Collaborative R&D schemes in the past have produced some lifechanging medications for patients, and so many more are yet to be created.
Between HCPs, pharma, patient advocates and governing bodies, it’s imperative that knowledge is shared so patient safety practices can grow to their full potential. Furthermore, by ensuring transparency of data around recent incidents/policy changes, we can facilitate faster learning and improvement to achieve a triple win for patients, pharma, and society. Research has shown that most patient safety errors occur due to flawed procedures, processes, and systems – all of which stand a greater chance of being resolved if we share data around what works and what doesn’t2. Once these data are shared, pharma can support the implementation of changes by informing HCPs and patients on lessons learned and steps taken. Pharma companies can promote this unified working by creating networks between themselves, HCPs and patients to help develop a common narrative and raise the profile of patient safety programmes. Collaborative R&D schemes in the past have produced some lifechanging medications for patients, and so many more are yet to be created.
- Communication
 In most areas of life there is a ‘knowing-doing gap’ between the knowledge of the provider and the consumer experience1 – the same is true in healthcare. When communication between consumer and provider becomes a two-way street, real changes happen. Pharma can catalyse this by implementing patient workshops into R&D (including at early stages) to learn what patients need and tell them what is available to them1. For example, involving patients in R&D and trials from the very start has shown to have many benefits, including reducing recruiting costs, faster progression to come to market, more effective patient-reported outcome measures and improved retention of participants5. The benefits to pharma are huge – by efficiently deploying their resources and have a greater impact on peoples’ lives, this translates to improved return on investment.
In most areas of life there is a ‘knowing-doing gap’ between the knowledge of the provider and the consumer experience1 – the same is true in healthcare. When communication between consumer and provider becomes a two-way street, real changes happen. Pharma can catalyse this by implementing patient workshops into R&D (including at early stages) to learn what patients need and tell them what is available to them1. For example, involving patients in R&D and trials from the very start has shown to have many benefits, including reducing recruiting costs, faster progression to come to market, more effective patient-reported outcome measures and improved retention of participants5. The benefits to pharma are huge – by efficiently deploying their resources and have a greater impact on peoples’ lives, this translates to improved return on investment.
- Transparency and trust
 Loss of trust is one of the biggest implications of poor patient safety, as has been seen with high-profile patient safety issues associated with various clinical trials in the past. The resulting loss of trust has implications for patients, HCPs and pharma as trust is essential between parties in relationships with unequal power dynamics6. Trust in people and organisations has been shown to be a powerful tool for change and is even thought to act as a placebo in some cases, thus explaining some of the varying effectiveness of traditional medicine7. As highlighted in The WHO Global Patient Safety Action Plan 2021-2030 by the strategic objective 7: “To amplify and disseminate good patient safety practices and learning at all levels, it is important to build partnerships and establish networks across the world. All collaborative initiatives and partnerships should be based on mutual respect and trust, with clear communication and a shared vision of the desired outcome. All patient safety partnerships should be multidisciplinary and multisectoral in composition, with strong cohesive coordination, co-planning and co-production as the foundation for success1.”
Loss of trust is one of the biggest implications of poor patient safety, as has been seen with high-profile patient safety issues associated with various clinical trials in the past. The resulting loss of trust has implications for patients, HCPs and pharma as trust is essential between parties in relationships with unequal power dynamics6. Trust in people and organisations has been shown to be a powerful tool for change and is even thought to act as a placebo in some cases, thus explaining some of the varying effectiveness of traditional medicine7. As highlighted in The WHO Global Patient Safety Action Plan 2021-2030 by the strategic objective 7: “To amplify and disseminate good patient safety practices and learning at all levels, it is important to build partnerships and establish networks across the world. All collaborative initiatives and partnerships should be based on mutual respect and trust, with clear communication and a shared vision of the desired outcome. All patient safety partnerships should be multidisciplinary and multisectoral in composition, with strong cohesive coordination, co-planning and co-production as the foundation for success1.”
Pharma companies can help to build these trusting networks by providing good data, admitting fault where necessary and working openly to avoid future errors. This may be difficult at first, but in the long run will improve business outcomes and success. Transparency of intentions is what helps to build trust between people and creates a lasting foundation for future advances. Overall, this will encourage bonds to form between groups to create a safer culture in healthcare.
- Education
The benefit of patient engagement is education. This can be two-directional – there is a lot to learn from each other. In patient safety, the key issue is that the general public are not ‘medication-wise’ and so tend to be passive in their healthcare, which heightens their risk of being subject to errors2. Through education on treatments and conditions, even on a basic level, this can be avoided. For example, teaching people about maximum doses, or explaining why something needs to be taken in a specific way to maximise efficacy. This will benefit pharma as it will help improve the safety record of their drugs and public perception of the industry, thus translating into better trust and more sales.
The WHO have also created a ‘Patient Safety Network’ to help strengthen and promote health education, which includes developing educational resources and supporting creation of, and relationships between, advocates at national/local/regional levels8. Pharma can support patient safety education by raising awareness of these programmes, helping to fund them or even producing educational materials themselves. Pharma can then use their connections with patient advocates and communities to help disseminate these resources for greater impact. In these ways and more, pharma can help patients to not only better understand safety but also realise when safety is at risk and flag to the appropriate parties before it’s too late.
Key takeaways for World Patient Safety Day 2021
- Patient safety needs major improvement across all therapeutic areas, with the help of multiple stakeholders and parties in the healthcare sector
- Death and injury from patient safety issues is very common but very avoidable.
- The solution lies in patient engagement, empowerment, and education.
- Pharma plays an integral role in all areas of patient safety and the more involved and active they are, the greater the benefits to them.
- By actively working to improve patient safety, we can achieve a triple win: better outcomes for patients, enhanced trust in pharma, and a safer, more transparent healthcare system in our society.
Read more about our work in Patient Engagement here
Follow our Twitter for more insights and updates from the world of patient engagement
References:
- Global Patient Safety Action Plan 2021–2030: towards eliminating avoidable harm in health care. Geneva: World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO.
- Medication Without Harm – Global Patient Safety Challenge on Medication Safety. Geneva: World Health Organization, 2017. Licence: CC BY-NC-SA 3.0 IGO.
- Pharma IQ [Internet]. Pharma IQ. 2021 [cited 27 August 2021]. Available from: https://www.pharma-iq.com/glossary/patient-safety
- Indicator Metadata Registry Details [Internet]. Who.int. 2021 [cited 10 September 2021]. Available from: https://www.who.int/data/gho/indicator-metadata-registry/imr-details/158
- Kersey O. Patient engagement in oncology R&D – pharmaphorum [Internet]. pharmaphorum. 2021 [cited 29 July 2021]. Available from: https://deep-dive.pharmaphorum.com/magazine/oncology-2021/oncology-rd-patient-engagement/
- Mechanic D, Meyer S. Concepts of trust among patients with serious illness. Social Science & Medicine. 2000;51(5):657-668.
- Branch WT. Is the therapeutic nature of the patient-physician relationship being undermined? Arch Intern Med. 2000;160:2257–2260.
- Patient Safety Network [internet]. PSN. 2021. [cited 27 August 2021]. Available from: https://psnet.ahrq.gov